Graphene
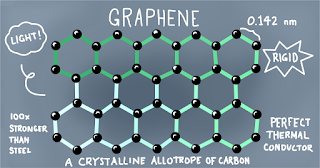
Graphene is an allotrope of carbon in the form of a two-dimensional, atomic-scale, hexagonal lattices in which one atom from each vertex.
Graphene can be described as a one-atom thick layer of graphite.
It is the strongest, thinnest material known to exist.
Each carbon atom is sp2 hybridized and it is bound to its the neighbors.
History of Graphene
One of the very first patents pertaining to the production of graphene was filed in October 2002 entitled, “ Nano-scaled Graphene Plates”.
Two years later, in 2004 Andre Geim and Kostya Novoselov at the university of Manchester extracted single-atom-thick crystallites from bulk graphite.
Geim and Novoselov received several awards for their pioneering research on graphene, notably the 2010 Nobel prize in physics.
Structure of Graphene
Graphene is a 2-dimensional network of the carbon atom.
These carbon atoms are bound to the plane by strong bond by strong bonds into a honeycomb array comprised of six-membered rings.
By stacking of these layers on top of each other, the well-known 3-dimensional graphite crystal is formed.
It is a basic building block for the graphitic material of all other dimensionalities.
It can be wrapped up into 0D fullerenes, rolled into 1D nanotubes or stacked into 3D graphite.
Chemical properties of graphene
Graphene is chemically the most reactive form of carbon.
The only form of carbon (and generally all solids material) in which each single atoms is in exposure for the chemical reaction from two side(due to the 2D structure).
Carbon atoms at the edge of graphene sheet have special chemical reactivity.
Graphene burns at very low temperature ( e.g. 350 oc) .
Graphene has the highest ratio of edge carbon (in comparison with similar materials such as carbon nanotube).
Electronic properties of graphene
It is a zero-overlap semimetal (with both holes and electrons as charge carriers) with very high electrical conductivity.
Electrons are able to flow through graphene more easily than through even copper.
The electrons travel through the graphene sheet as if they carry no mass, as fast as just one hundredth that of the speed of light.
High charge carrier mobility, for which value of 10,000 cm2/Vs, in some cases even 200,000 cm2/Vs were reported.
Mechanical properties of Graphene
To calculate the strength of graphene, scientists used a technique called Atomic force microscopy.
It was found that graphene is harder than diamond and about 300 times harder than steel.
The tensile strength of graphene exceeds 1 TPa.
It is stretchable up to 205 of its initial length.
It is expected that graphene’s mechanical properties will find application into the making of a new generation of super-strong composite material and along combined with its optical properties, making a flexible display.
Applications
Graphene could soon be used to analyze DNA at a record-breaking pace.
Graphene has a high carrier mobility, as well as low noise allowing it to be used as the channel in a field effect transistor.
Graphene’s high electrical conductivity and high optical transparency make it a candidate for the transparent conducting electrode.
Graphenes are used for many applications some of them are given below
Solar cells, energy storage devices, IR detectors, single-molecule gas detection, energy harvesting, composite materials, chemical sensor etc.



Nice topic selected for blogspot
ReplyDeletethanks
ReplyDeleteThis comment has been removed by the author.
ReplyDelete